Background
Photovoltaic solar cells are thin silicon disks that convert sunlight into electricity. These disks act as energy sources for a wide variety of uses, including: calculators and other small devices; telecommunications; rooftop panels on individual houses; and for lighting, pumping, and medical refrigeration for villages in developing countries. Solar cells in the form of large arrays are used to power satellites and, in rare cases, to provide electricity for power plants.
When research into electricity began and simple batteries were being made and studied, research into solar electricity followed amazingly quickly. As early as 1839, Antoine-Cesar Becquerel exposed a chemical battery to the sun to see it produce voltage. This first conversion of sunlight to electricity was one percent efficient. That is, one percent of the incoming sunlight was converted into electricity. Willoughby Smith in 1873 discovered that selenium was sensitive to light; in 1877 Adams and Day noted that selenium, when exposed to light, produced an electrical current. Charles Fritts, in the 1880s, also used gold-coated selenium to make the first solar cell, again only one percent efficient. Nevertheless, Fritts considered his cells to be revolutionary. He envisioned free solar energy to be a means of decentralization, predicting that solar cells would replace power plants with individually powered residences.
With Albert Einstein’s explanation in 1905 of the photoelectric effect—metal absorbs energy from light and will retain that energy until too much light hits it—hope soared anew that solar electricity at higher efficiencies would become feasible. Little progress was made, however, until research into diodes and transistors yielded the knowledge necessary for Bell scientists Gordon Pearson, Darryl Chapin, and Cal Fuller to produce a silicon solar cell of four percent efficiency in 1954.
Further work brought the cell’s efficiency up to 15 percent. Solar cells were first used in the rural and isolated city of Americus, Georgia as a power source for a telephone relay system, where it was used successfully for many years.
A type of solar cell to fully meet domestic energy needs has not as yet been developed, but solar cells have become successful in providing energy for artificial satellites. Fuel systems and regular batteries were too heavy in a program where every ounce mattered. Solar cells provide more energy per ounce of weight than all other conventional energy sources, and they are cost-effective.
Only a few large scale photovoltaic power systems have been set up. Most efforts lean toward providing solar cell technology to remote places that have no other means of sophisticated power. About 50 megawatts are installed each year, yet solar cells provide only about .1 percent of all electricity now being produced. Supporters of solar energy claim that the amount of solar radiation reaching the Earth’s surface each year could easily provide all our energy needs several times over, yet solar cells have a long way to go before they fulfill Charles Fritts’s dream of free, fully accessible solar electricity.
(To make solar cells, the raw materials—silicon dioxide of either quartzite gravel or crushed quartz—are first placed into an electric arc furnace, where a carbon arc is applied to release the oxygen. The products are carbon dioxide and molten silicon. At this point, the silicon is still not pure enough to be used for solar cells and requires further purification)
Raw Materials
The basic component of a solar cell is pure silicon, which is not pure in its natural state. Pure silicon is derived from such silicon dioxides as quartzite gravel (the purest silica) or crushed quartz. The resulting pure silicon is then doped (treated with) with phosphorous and boron to produce an excess of electrons and a deficiency of electrons respectively to make a semiconductor capable of conducting electricity. The silicon disks are shiny and require an anti-reflective coating, usually titanium dioxide.
The solar module consists of the silicon semiconductor surrounded by protective material in a metal frame. The protective material consists of an encapsulant of transparent silicon rubber or butyryl plastic (commonly used in automobile windshields) bonded around the cells, which are then embedded in ethylene vinyl acetate. A poly-ester film (such as mylar or tedlar) makes up the backing. A glass cover is found on terrestrial arrays, a lightweight plastic cover on satellite arrays. The electronic parts are standard and consist mostly of copper. The frame is either steel or aluminum. Silicon is used as the cement to put it all together.
The Manufacturing Process
Purifying the silicon
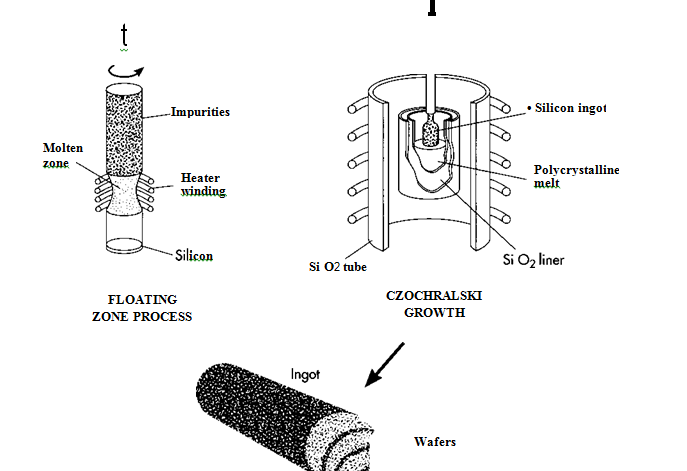
1 The silicon dioxide of either quartzite gravel or crushed quartz is placed into an electric arc furnace. A carbon arc is then applied to release the oxygen. The products are carbon dioxide and molten silicon. This simple process yields silicon with one percent impurity, useful in many industries but not the solar cell industry.
2 The 99 percent pure silicon is purified even further using the floating zone technique. A rod of impure silicon is passed through a heated zone several times in the same direction. This procedure “drags” the impurities toward one end with each pass. At a specific point, the silicon is deemed pure, and the impure end is removed.
Making single crystal silicon
3 Solar cells are made from silicon boules, polycrystalline structures that have the atomic structure of a single crystal. The most commonly used process for creating the boule is called the Czochralski method. In this process, a seed crystal of silicon is dipped into melted polycrystalline silicon. As the seed crystal is withdrawn and rotated, a cylindrical ingot or “boule” of silicon is formed. The ingot withdrawn is unusually pure, because impurities tend to remain in the liquid.
Making silicon wafers
4 From the boule, silicon wafers are sliced one at a time using a circular saw whoseinner diameter cuts into the rod, or many at once with a multiwire saw. (A diamond saw produces cuts that are as wide as the wafer—.5 millimeter thick.) Only about one-half of the silicon is lost from the boule to the finished circular wafer—more if the wafer is then cut to be rectangular or hexagonal. Rectangular or hexagonal wafers are sometimes used in solar cells because they can be fitted together perfectly, thereby utilizing all available space on the front surface of the solar cell. C The wafers are then polished to remove ^ saw marks. (It has recently been found that rougher cells absorb light more effectively, therefore some manufacturers have chosen not to polish the wafer.)
(After the initial purification, the silicon is further refined in a floating zone process. In this process, a silicon rod is passed through a heated zone several times, which serves to “drag” the impurities toward one end of the rod. The impure end can then be removed. Next, a silicon seed crystal is put into a Czochralski growth apparatus, where it is dipped into melted polycrystalline silicon. The seed crystal rotates as it is withdrawn, forming a cylindrical ingot of very pure silicon. Wafers are then sliced out of the ingot.)
Doping
The traditional way of doping (adding impurities to) silicon wafers with boron and phosphorous is to introduce a small amount of boron during the Czochralski process in step #3 above. The wafers are then sealed back to back and placed in a furnace to be heated to slightly below the melting point of silicon (2,570 degrees Fahrenheit or 1, 410 degrees Celsius) in the presence of phosphorous gas. The phosphorous atoms “burrow” into the silicon, which is more porous because it is close to becoming a liquid. The temperature and time given to the process is carefully controlled to ensure a uniform junction of proper depth.
A more recent way of doping silicon with phosphorous is to use a small particle accelerator to shoot phosphorous ions into the ingot. By controlling the speed of the ions, it is possible to control their penetrating depth. This new process, however, has generally not been accepted by commercial manufacturers.
Placing electrical contacts
7 Electrical contacts connect each solar cell to another and to the receiver of produced current. The contacts must be very thin (at least in the front) so as not to block sunlight to the cell. Metals such as palladium/silver, through a photoresist, silkscreened, or merely deposited on the exposed portion of cells that have been partially covered with wax. All three methods involve a system in which the part of the cell on which a contact is not desired is protected, while the rest of the cell is exposed to the metal.
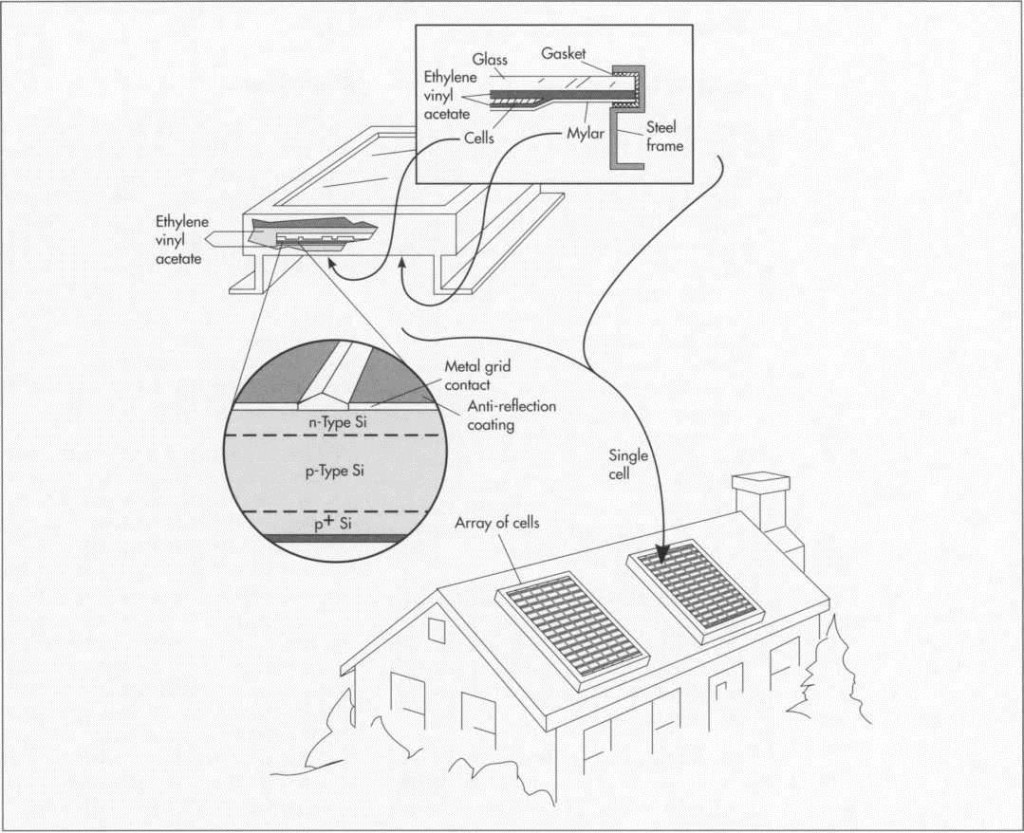
(This illustration shows the makeup of a typical solar cell. The cells are encapsulated in ethylene vinyl acetate and placed in a metal frame that has a mylar backsheet and glass cover.)
8 After the contacts are in place, thin strips (“fingers”) are placed between cells. The most commonly used strips are tin-coated copper.
The anti-reflective coating
9 Because pure silicon is shiny, it can reflect up to 35 percent of the sunlight. To reduce the amount of sunlight lost, an anti-reflective coating is put on the silicon wafer. The most commonly used coatings are titanium dioxide and silicon oxide, though others are used. The material used for coating is either heated until its molecules boil off and travel to the silicon and condense, or the material undergoes sputtering. In this process, a high voltage knocks molecules off the material and deposits them onto the silicon at the opposite electrode. Yet another method is to allow the silicon itself to react with oxygen- or nitrogen-containing gases to form silicon dioxide or silicon nitride. Commercial solar cell manufacturers use silicon nitride.
Encapsulating the cell
The finished solar cells are then encapsulated; that is, sealed into silicon rubber or ethylene vinyl acetate. The encapsulated solar cells are then placed into an aluminum frame that has a mylar or tedlar backsheet and a glass or plastic cover.
Quality Control
Quality control is important in solar cell manufacture because discrepancy in the many processes and factors can adversely affect the overall efficiency of the cells. The primary research goal is to find ways to improve the efficiency of each solar cell over a longer lifetime. The Low Cost Solar Array Project (initiated by the United States Department of Energy in the late 1970s) sponsored private research that aimed to lower the cost of solar cells. The silicon itself is tested for purity, crystal orientation, and resistivity. Manufacturers also test for the presence of oxygen (which affects its strength and resistance to warp) and carbon (which causes defects). Finished silicon disks are inspected for any damage, flaking, or bending that might have occurred during sawing, polishing, and etching.
During the entire silicon disk manufacturing process, the temperature, pressure, speed, and quantities of dopants are continuously monitored. Steps are also taken to ensure that impurities in the air and on working surfaces are kept to a minimum.
The completed semiconductors must then undergo electrical tests to see that the current, voltage, and resistance for each meet appropriate standards. An earlier problem with solar cells was a tendency to stop working when partially shaded. This problem has been alleviated by providing shunt diodes that reduce dangerously high voltages to the cell. Shunt resistance must then be tested using partially shaded junctions.
An important test of solar modules involves providing test cells with conditions and intensity of light that they will encounter under normal conditions and then checking to see that they perform well. The cells are also exposed to heat and cold and tested against vibration, twisting, and hail.
The final test for solar modules is field site testing, in which finished modules are placed where they will actually be used. This provides the researcher with the best data for determining the efficiency of a solar cell under ambient conditions and the solar cell’s effective lifetime, the most important factors of all.
The Future
Considering the present state of relatively expensive, inefficient solar cells, the future can only improve. Some experts predict it will be a billion-dollar industry by the year 2000. This prediction is supported by evidence of more rooftop photovoltaic systems being developed in such countries as Japan, Germany, and Italy. Plans to begin the manufacture of solar cells have been established in Mexico and China. Likewise, Egypt, Botswana, and the Philippines (all three assisted by American companies) are building plants that will manufacture solar cells.
Most current research aims for reducing solar cell cost or increasing efficiency. Innovations in solar cell technology include developing and manufacturing cheaper alternatives to the expensive crystalline silicon cells. These alternatives include solar windows that mimic photosynthesis, and smaller cells made from tiny, amorphous silicon balls. Already, amorphous silicon and polycrystalline silicon are gaining popularity at the expense of single crystal silicon. Additional innovations including minimizing shade and focusing sunlight through prismatic lenses. This involves layers of different materials (notably, gallium arsenide and silicon) that absorb light at different frequencies, thereby increasing the amount of sunlight effectively used for electricity production.
A few experts foresee the adaptation of hybrid houses; that is, houses that utilize solar water heaters, passive solar heating, and solar cells for reduced energy needs. Another view concerns the space shuttle placing more and more solar arrays into orbit, a solar power satellite that beams power to Earth solar array farms, and even a space colony that will manufacture solar arrays to be used on Earth.
Where To Learn More
Books
Bullock, Charles E. and Peter H. Grambs. Solar Electricity: Making the Sun Work for You. Monegon, Ltd., 1981.
Komp, Richard J. Practical Photovoltaics. Aatec Publications, 1984.
Making and Using Electricity from the Sun. Tab Books, 1979.
Periodicals
Crawford, Mark. “DOE’s Born-Again Solar Energy Plan,” Science. March 23, 1990, pp. 1403-1404.
“Waiting for the Sunrise,” Economist. May 19, 1990, pp. 95+.
Edelson, Edward. “Solar Cell Update,” Popular Science. June, 1992, p. 95.
Murray, Charles J. “Solar Power’s Bright Hope,” Design News. March 11, 1991, p. 30.