Background
Gold, recognizable by its yellowish cast, is one of the oldest metals used by humans. As far back as the Neolithic period, humans have collected gold from stream beds, and the actual mining of gold can be traced as far back as 3500 B.C., when early Egyptians (the Sumerian culture of Mesopotamia) used mined gold to craft elaborate jewelry, religious artifacts, and utensils such as goblets.
Gold’s aesthetic properties combined with its physical properties have long made it a valuable metal. Throughout history, gold has often been the cause of both conflict and adventure: the destruction of both the Aztec and Inca civilizations, for instance, and the early American gold rushes to Georgia, California, and Alaska.
The largest deposit of gold can be found in South Africa in the Precambrian Witwatersrand Conglomerate. This deposit of gold ore is hundreds of miles across and more than two miles deep. It is estimated that two-thirds of the gold mined comes from South Africa. Other major producers of gold include Australia, the former Soviet Union, and the United States (Arizona, Colorado, California, Montana, Nevada, South Dakota, and Washington).
About 65 percent of processed gold is used in the arts industry, mainly to make jewelry. Besides jewelry, gold is also used in the electrical, electronic, and ceramics industries. These industrial applications have grown in recent years and now occupy an estimated 25 percent of the gold market. The remaining percentage of mined gold is used to make a type of ruby colored glass called purple of Cassius, which is applied to office building windows to reduce the heat in the summer, and to mirrors used in space and in electroscopy so that they reflect the infrared spectrum.
Physical Characteristics
Gold, whose chemical symbol is Au, is malleable, ductile, and sectile, and its high thermal and electrical conductivity as well as its resistance to oxidation make its uses innumerable. Malleability is the ability of gold and other metals to be pressed or hammered into thin sheets, 10 times as thin as a sheet of paper. These sheets are sometimes evaporated onto glass for infrared reflectivity, molded as fillings for teeth, or used as a coating or plating for parts. Gold’s ability to be drawn into thin wire (ductility) enables it to be deposited onto circuits such as transistors and to be used as an industrial solder and brazing alloy. For example, gold wire is often used for integrated circuit electrical connections, for orthodontic and prosthetic appliances, and in jet engine fabrication.
Gold’s one drawback for use in industry is that it is a relatively soft metal (sectile). To combat this weakness, gold is usually alloyed with another member of the metal family such as silver, copper, platinum, or nickel. Gold alloys are measured by karats (carats). A karat is a unit equal to 1/24 part of pure gold in an alloy. Thus, 24 karat (24K) gold is pure gold, while 18 karat gold is 18 parts pure gold to 6 parts other metal.
Extraction and Refining
Gold is usually found in a pure state; however, it can also be extracted from silver, copper, lead and zinc. Seawater can also contain gold, but in insufficient quantities to be profitably extracted—up to one-fortieth (1/40) of a grain of gold per ton of water. Gold is generally found in two types of deposits: lode (vein) or placer deposits; the mining technique used to extract the gold depends upon the type of deposit. Once extracted, the gold is refined with one of four main processes: floatation, amalgamation, cyanidation, or carbon-in-pulp. Each process relies on the initial grinding of the gold ore, and more than one process may be used on the same batch of gold ore.
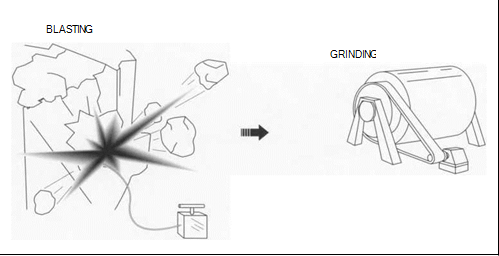
(Gold is generally found in two types of deposits: lode (vein) or placer deposits. It is usually extracted from lode deposits by drilling or blasting, whereas placer deposits require hydraulic mining, dredging, or power shoveling. Once extracted, the gold ore is pulverized to prepare it for refining.)
Mining
1 ln lode or vein deposits, the gold is mixed with another mineral, often quartz, in a vein that has filled a split in the surrounding rocks. Gold is obtained from lode deposits by drilling, blasting, or shoveling the surrounding rock. Lode deposits often run deep underground. To mine underground, miners dig shafts into the ground along the vein. Using picks and small explosives, they then remove the gold ore from the surrounding rock. The gold ore is then gathered up and taken to a mill for refinement.
2 Placer deposits contain large pieces of gold ore (nuggets) and grains of gold that have been washed downstream from a lode deposit and that are usually mixed with sand or gravel. The three main methods used to mine placer deposits are hydraulic mining, dredging, and power shoveling. All methods of placer deposit mining use gravity as the basic sorting force. In the first method, a machine called a “hydraulic giant” uses a high pressure stream of water to knock the gold ore off of banks containing the ore. The gold ore is then washed down into sluices or troughs that have grooves to catch the gold.
Dredging and power shoveling involve the same techniques but work with different size buckets or shovels. In dredging, buckets on a conveyor line scoop sand, gravel, and gold ore from the bottom of streams. In power shoveling, huge machines act like shovels and scoop up large quantities of gold-bearing sand and gravel from stream beds. Hydraulic mining and dredging are outlawed in many countries because they are environmentally destructive to both land and streams.
Grinding
3 Once the gold ore has been mined, it usually is washed and filtered at the mine as a preliminary refinement technique. It is then shipped to mills, where it is first combined with water and ground into smaller chunks. The resulting mixture is then further ground in a ball mill—a rotating cylindrical vessel that uses steel balls to pulverize the ore.
Separating the gold from the ore
(Floatation, cyanidation, and the carbon-in-pulp method are 3 processes used to refine gold. They can be used alone or in combination with one another)
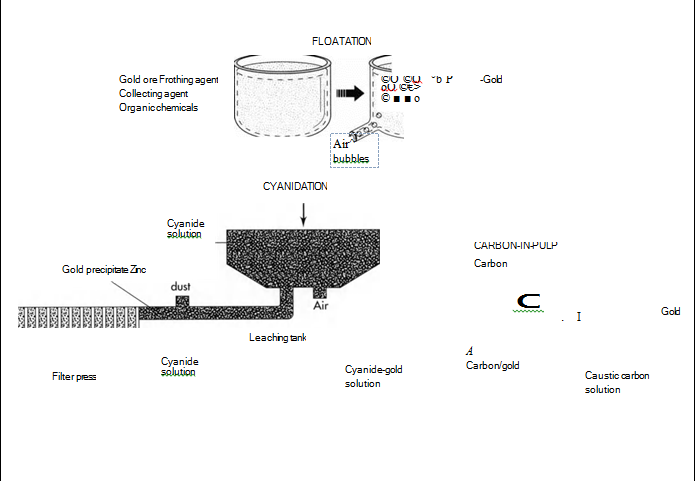
4 The gold is then separated from the ore using one of several methods. Floatation involves the separation of gold from its ore by using certain chemicals and air. The finely ground ore is dumped into a solution that contains a frothing agent (which causes the water to foam), a collecting agent (which bonds onto the gold, forming an oily film that sticks to air bubbles), and a mixture of organic chemicals (which keep the other contaminants from also bonding to the air bubbles). The solution is then aerated—air bubbles are blown in—and the gold attaches to the air bubbles. The bubbles float to the top, and the gold is skimmed off.
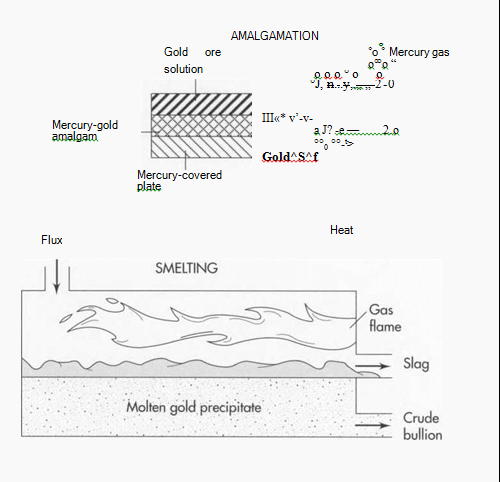
Cyanidation also involves using chemicals to separate the gold from its contaminants. In this process, the ground ore is placed in a tank containing a weak solution of cyanide. Next, zinc is added to the tank, causing a chemical reaction in which the end result is the precipitation (separation) of the gold from its ore. The gold precipitate is then separated from the cyanide solution in a filter press. A similar method is amalgamation, which uses the same process with different chemicals. First, a solution carries the ground ore over plates covered with mercury. The mercury attracts the gold, forming an alloy called an amalgam. The amalgam is then heated, causing the mercury to boil off as a gas and leaving behind the gold. The mercury is collected, recycled and used again in the same process.
The carbon-in-pulp method also uses cyanide, but utilizes carbon instead of zinc to precipitate the gold. The first step is to mix the ground ore with water to form a pulp. Next, cyanide is added to dissolve the gold, and then carbon is added to bond with the gold. After the carbon particles are removed from the pulp, they are placed in a hot caustic (corrosive) carbon solution, which separates the gold from the carbon.
(Two other methods of gold refining are amalgamation and smelting. In amalgamation, the gold ore is dissolved in solution and passed over mercury-covered plates to form a gold/mercury amalgam. When the amalgam is heated, the mercury boils off as a gas and leaves behind the gold. In smelting, the gold is heated with a chemical substance called “flux.” The flux bonds with the contaminants and floats on top of the gold. The flux-contaminant mixture (slag) is hauled away, leaving a gold precipitate.)
5 If the gold is still not pure enough, it can be smelted. Smelting involves heating the gold with a chemical substance called flux. The flux bonds with the contaminants and floats on top of the melted gold. The gold is then cooled and allowed to harden in molds, and the flux-contaminant mixture (slag) is hauled away as a solid waste.
The Future
Because gold is a finite resource, its longterm future is limited. In the short term, however, it will continue to find widespread use in jewelry and in industrial applications, especially in the electronics field. In the last few years, several companies have focused on extracting gold from sulphide ore rather than oxide ore. Previous techniques made such extraction difficult and expensive, but a newer technique called bioleaching has made extraction more feasible. The process involves combining the sulphide ore with special bacteria that “eat” the ore or break it down into a more manageable form.
Where To Learn More
Books
Coombs, Charles. Gold and Other Precious Metals. Morrow Publishing, 1981.
Gasparrini, Claudia. Gold & Other Precious Metals: From Ore to Market. Springer-Verlag, 1993.
Green, Timothy. The World of Gold. Walker Publishing, 1968.
Hawkins, Clint. Gold c£ Lead. Harper- Collins, 1993.
Lye, Keith. Spotlight on Gold. Rourke Enterprises, 1988.