Background
Unlike many other cosmetics that have a history of hundreds or even thousands of years, nail polish (or lacquer, or enamel) is almost completely an invention of twentieth century technology. Nail coverings were not unknown in ancient times—the upper classes of ancient Egypt probably used henna to dye both hair and fingernails—but essentially, its composition, manufacture and handling reflect developments in modem chemical technology.
Modem nail polish is sold in liquid form in small bottles and is applied with a tiny brash. Within a few minutes after application, the substance hardens and forms a shiny coating on the fingernail that is both water- and chip- resistant. Generally, a coating of nail polish may last several days before it begins to chip and fall off. Nail polish can also be removed manually by applying nail polish “remover,” a substance designed to break down and dissolve the polish.
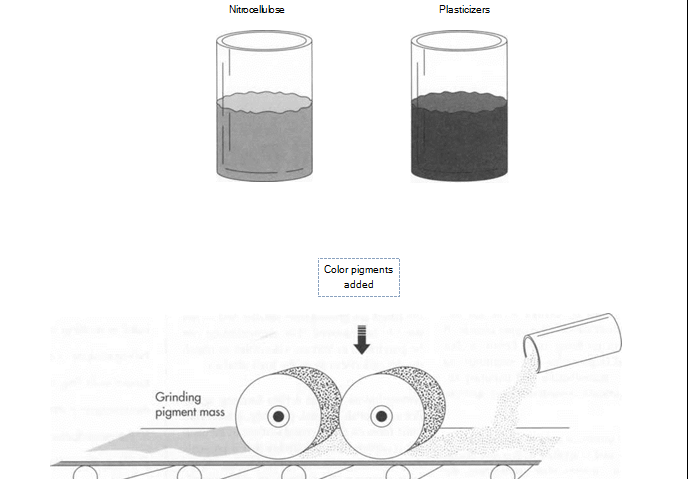
(Nail polish is made by combining nitrocellulose and plasticizers with color pigments. The mixing is done in a “two-roll” differential speed mill, which grinds the pigment between a pair of rollers that are able to work with increasing speed as the pigment is ground down. The goal is to produce fine dispersion of the color.)
Raw Materials
There is no single formula for nail polish. There are, however, a number of ingredient types that are used. These basic components include: film forming agents, resins and plasticizers, solvents, and coloring agents. The exact formulation of a nail polish, apart from being a corporate secret, greatly depends upon choices made by chemists and chemical engineers in the research and development phase of manufacturing. Additionally, as chemicals and other ingredients become accepted or discredited for some uses, adjustments are made. For example, formaldehyde was once frequently used in polish production, but now it is rarely used.
The primary ingredient in nail polish is nitro-cellulose (cellulose nitrate) cotton, a flammable and explosive ingredient also used in making dynamite. Nitrocellulose is a liquid mixed with tiny, near-microscopic cotton fibers. In the manufacturing process, the cotton fibers are ground even smaller and do not need to be removed. The nitrocellulose can be purchased in various viscosities to match the desired viscosity of the final product.
Nitrocellulose acts as a film forming agent. For nail polish to work properly, a hard film must form on the exposed surface of the nail, but it cannot form so quickly that it prevents the material underneath from drying. (Consider commercial puddings or gelatin products that dry or film on an exposed surface and protect the moist product underneath.) By itself or used with other functional ingredients, the nitrocellulose film is brittle and adheres poorly to nails.
Manufacturers add synthetic resins and plasticizers (and occasionally similar, natural products) to their mixes to improve flexibility, resistance to soap and water, and other qualities; older recipes sometimes even used nylon for this purpose. Because of the number of desired qualities involved, however, there is no single resin or combination of resins that meets every specification. Among the resins and plasticizers in use today are castor oil, amyl and butyl stearate, and mixes of glycerol, fatty acids, and acetic acids.
The colorings and other components of nail polish must be contained within one or more solvents that hold the colorings and other materials until the polish is applied. After application, the solvent must be able to evaporate. In many cases, the solvent also acts a plasticizer. Butyl stearate and acetate compounds are perhaps the most common.
Finally, the polish must have a color. Early polishes used soluble dyes, but today’s product contains pigments of one type or another. Choice of pigment and its ability to mix well with the solvent and other ingredients is essential to producing a good quality product.
Nail polish is a “suspension” product, in which particles of color can only be held by the solvent for a relatively short period of time, rarely more than two or three years. Shaking a bottle of nail polish before use helps to restore settled particles to the suspension; a very old bottle of nail polish may have so much settled pigment that it can never be restored to the solvent. The problem of settling is perhaps the most difficult to be addressed in the manufacturing process.
In addition to usual coloring pigments, other ‘color tones can be added depending upon the color, tone, and hue of the desired product. Micas (tiny reflective minerals), also used in lipsticks, are a common additive, as is “pearl” or “fish scale” essence. “Pearl” or “guanine” is literally made from small fish scales and skin, suitably cleaned, and mixed with solvents such as castor oil and butyl acetate. The guanine can also be mixed with gold, silver, and bronze tones.
Pigment choices are restricted by the federal Food and Drug Administration (FDA), which maintains lists of pigments considered acceptable and others that are dangerous and cannot be used. Manufacturing plants are inspected regularly, and manufacturers must be able to prove they are using only FDA approved pigments. Since the FDA lists of acceptable and unacceptable pigments change with new findings and reexaminations of colors, manufacturers occasionally have to reformulate a polish formula.
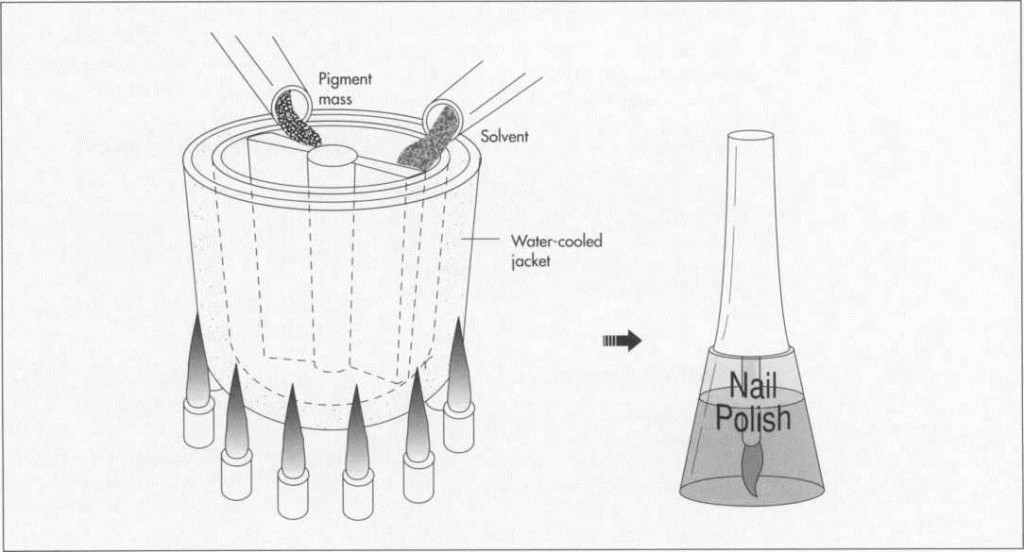
(Once the pigment mass is prepared, it is mixed with solvent in a stainless steel kettle. The kettle has a water-jacket to facilitate cooling of the mixture.)
The Manufacturing Process
Early methods of making nail polish used a variety of methods that today look charmingly amateurish. One common technique was to mix cleaned scraps of movie film and other cellulose with alcohol and castor oil and leave the mixture to soak overnight in a covered container. The mixture was then strained, colored, and perfumed. Though recognizable as nail polish, the product was far from what we have available today. The modem manufacturing process is a very sophisticated operation utilizing highly skilled workers, advanced machinery, and even robotics. Today’s consumers expect a nail polish to apply smoothly, evenly, and easily; to set relatively quickly; and to be resistant to chipping and peeling. In addition, the polish should be dermatologically innocuous.
Mixing the pigment with nitrocellulose and plasticizer
1 The pigments are mixed with nitrocellulose and plasticizer using a “two-roll” differential speed mill. This mill grinds the pigment between a pair of rollers that are able to work with increasing speed as the pigment is ground down. The goal is to produce fine dispersion of the color. A variation of this mill is the Banbury Mixer (used also in the production of rubber for rubber bands).
2 When properly and fully milled, the mixture is removed from the mill in sheet form and then broken up into small chips for mixing with the solvent. The mixing is performed in stainless steel kettles that can hold anywhere from 5 to 2,000 gallons. Stainless steel must be used because the nitrocellulose is extremely reactive in the presence of iron. The kettles are jacketed so that the mixture can be cooled by circulating cold water or another liquid around the outside of the kettle. The temperature of the kettle, and the rate of cooling, are controlled by both computers and technicians.
This step is performed in a special room or area designed to control the hazards of fire and explosion. Most modern factories perform this step in an area with walls that will close in if an alarm sounds and, in the event of explosion, with ceilings that will safely blow off without endangering the rest of the structure.
Adding other ingredients
3 Materials are mixed in computerized, closed kettles. At the end of the process, the mix is cooled slightly before the addition of such other materials as perfumes and moisturizers.
4 The mixture is then pumped into smaller, 55 gallon drums, and then trucked to a production line. The finished nail polish is pumped into explosion proof pumps, and then into smaller bottles suitable for the retail market.
Quality Control
Extreme attention to quality control is essential throughout the manufacturing process. Not only does quality control increase safety in the process, but it is the only way that a manufacturer can be assured of consumer confidence and loyalty. A single bottle of poor quality polish can lose a customer forever. Regardless of quality control, however, no single nail polish is perfect; the polish always represents a chemical compromise between what is desired and what the manufacturer is able to produce.
The nail polish is tested throughout the manufacturing process for several important factors (drying time, smoothness of flow, gloss, hardness, color, abrasion resistance, etc.). Subjective testing, where the mixture or final product is examined or applied, is ongoing. Objective, laboratory testing of samples, though more time consuming, is also necessary to ensure a usable product. Laboratory tests are both complicated and unforgiving, but no manufacturer would do without them.
The Future
Perhaps the major problem with nail polishes—from the consumer’s point of view— is the length of the drying time. Various methods of producing fast-drying polish have recently been patented, and these methods, along with others that are still being developed, may result in marketable products. Of all the different types of cosmetics, nail polish is the one that is most likely to continue to be positively affected by advancements and developments in the chemistry field.
Where To Learn More
Books
Balsam, M. S., ed. Cosmetics: Science & Technology. Krieger Publishing, 1991.
Chemistry of Soap, Detergents, & Cosmetics. Flinn Scientific, 1989.
Flick, Ernest W. Cosmetic & Toiletry Formulations. 2nd ed., Noyes Press, 1992.
Meyer, Carolyn. Being Beautiful: The Story of Cosmetics From Ancient Art to Modern Science. William Morrow and Company, 1977.
Wells, F. V. and Irwin I. Lubowe, M.D., eds. Cosmetics and The Skin. Reinhold Publishing Corp., 1964.
Periodicals
Andrews, Edmund L. “Patents: A Nail Polish That Dries Fast.” New York Times, March 7, 1992, p. 40.
Andrews, Edmund L. “Patents: Quick-Dry Coating for Nail Polish.” New York Times, June 13, 1992, p. 36.
“Makeup Formulary.” Cosmetics & Toiletries, April, 1986, pp. 103-22.
Ikeda, T., T. Kobayashi, and C. Tanaka. “Development of Highly Safe Nail Enamel.” Cosmetics & Toiletries, April, 1988, pp. 59¬60+.
Schlossman, Mitchell L. “Nail Cosmetics.” Cosmetics & Toiletries, April, 1986, pp. 23¬4+