Today, Americans alone consume 16,000 tons of aspirin tablets a year, equaling 80 million pills, and we spend about $2 billion a year for non-prescription pain relievers, many of which contain aspirin or similar drugs. 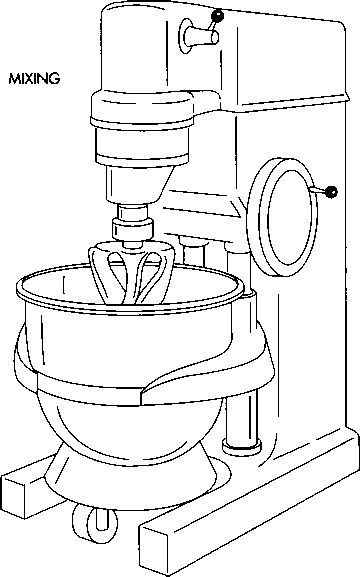
Lubricant Corn starch
SCREENING
Background
Aspirin is one of the safest and least expensive pain relievers on the marketplace. While other pain relievers were discovered and manufactured before aspirin, they only gained acceptance as over-the-counter drugs in Europe and the United States after aspirin’s success at the turn of the twentieth century. Today, Americans alone consume 16,000 tons of aspirin tablets a year, equaling 80 million pills, and we spend about $2 billion a year for non-prescription pain relievers, many of which contain aspirin or similar drugs. Currently, the drug is available in several dosage forms in various concentrations from .0021 to .00227 ounces (60 to 650 milligrams), but the drug is most widely used in tablet form. Other dosage forms include capsules, caplets, suppositories and liquid elixir. Aspirin can be used to fight a host of health problems: cerebral thromboses (with less than one tablet a day); general pain or fever (two to six tablets a day; and diseases such as rheumatic fever, gout, and rheumatoid arthritis. The drug is also beneficial in helping to ward off heart attacks. In addition, biologists use aspirin to interfere with white blood cell action, and molecular biologists use the drug to activate genes. The wide range of effects that aspirin can produce made it difficult to pinpoint how it actually works, and it wasn’t until the 1970s that biologists hypothesized that aspirin and related drugs (such as ibuprofen) work by inhibiting the synthesis of certain hormones that cause pain and inflammation. Since then, scientists have made further progress in understanding how aspirin works. They now know, for instance, that aspirin and its relatives actually prevent the growth of cells that cause inflammation.
History
The compound from which the active ingredient in aspirin was first derived, salicylic acid, was found in the bark of a willow tree in 1763 by Reverend Edmund Stone of Chipping-Norton, England. (The bark from the willow tree—Salix Alba—contains high levels of salicin, the glycoside of salicylic acid.) Earlier accounts indicate that Hippocrates of ancient Greece used willow leaves for the same purpose—to reduce fever and relieve the aches of a variety of illnesses. During the 1800s, various scientists extracted salicylic acid from willow bark and produced the compound synthetically. Then, in 1853, French chemist Charles F. Gerhardt synthesized a primitive form of aspirin, a derivative of salicylic acid. In 1897 Felix Hoffmann, a German chemist working at the Bayer division of I.G. Farber, discovered a better method for synthesizing the drug. Though sometimes Hoffmann is improperly given credit for the discovery of aspirin, he did understand that aspirin was an effective pain reliever that did not have the side effects of salicylic acid (it burned throats and upset stomachs). Bayer marketed aspirin beginning in 1899 and dominated the production of pain relievers until after World War I, when Sterling Drug bought German-owned Bayer’s New most effective lubricant for hard aspirin tablets. Chewable aspirin tablets contain different diluents, such as mannitol, lactose, sorbitol, sucrose, and inositol, which allow the tablet to dissolve at a faster rate and give the drug a pleasant taste. In addition, flavor agents, such as saccharin, and coloring agents are added to chewable tablets. The colorants currently approved in the United States include: FD&C Yellow No. 5, FD&C Yellow No. 6, FD&C Red No.3, FD&C Red No. 40, FD&C Blue No. 1, FD&C Blue No. 2, FD&C Green No. 3, a limited number of D&C colorants, and iron oxides.
The Manufacturing Process
Aspirin tablets are manufactured in different shapes. Their weight, size, thickness, and hardness may vary depending on the amount of the dosage. The upper and lower surfaces of the tablets may be flat, round, concave, or convex to various degrees. The tablets may also have a line scored down the middle of the outer surface, so the tablets can be broken in half, if desired. The tablets may be engraved with a symbol or letters to identify the manufacturer. Aspirin tablets of the same dosage amount are manufactured in batches. After careful weigh¬ing, the necessary ingredients are mixed and compressed into units of granular mixture called slugs. The slugs are then filtered to remove air and lumps, and are compressed again (or punched) into numerous individual tablets. (The number of tablets will depend on the size of the batch, the dosage amount, and the type of tablet machine used.) Documentation on each batch is kept throughout the manufacturing process, and finished tablets undergo several tests before they are bottled and packaged for distribution.
The procedure for manufacturing hard aspirin tablets, known as dry-granulation or slugging, is as follows: Weighing
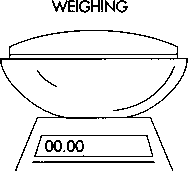
1 The com starch, the active ingredient, and the lubricant are weighed separately in sterile canisters to determine if the ingredients meet pre-determined specifications for the batch size and dosage amount.
Mixing
2 The corn starch is dispensed into cold purified water, then heated and stirred until a translucent paste forms. The corn starch, the active ingredient, and part of the lubricant are next poured into one sterile canister, and the canister is wheeled to a mixing machine called a Glen Mixer. Mixing blends the ingredients as well as expels air from the mixture.
3 The mixture is then mechanically separated into units, which are generally from 7/8 to 1 inches (2.22 to 2.54 centimeters) in size. These units are called slugs.
Dry screening
4 Next, small batches of slugs are forced through a mesh screen by a hand-held stainless steel spatula. Large batches in sizable manufacturing outlets are filtered through a machine called a Fitzpatrick mill. The remaining lubricant is added to the mixture, which is blended gently in a rotary granulator and sifter. The lubricant keeps the mixture from sticking to the tablet machine during the compression process.
Compression
5 The mixture is compressed into tablets either by a single-punch machine (for small batches) or a rotary tablet machine (for large scale production). The majority of single-punch machines are power-driven, but hand-operated models are still available. On single-punch machines, the mixture is fed into one tablet mold (called a dye cavity) by a feed shoe, as follows:
• The feed shoe passes over the dye cavity and releases the mixture. The feed shoe then retracts and scrapes all excess mixture away from the dye cavity.
• A punch—a short steel rod—the size of the dye cavity descends into the dye, compressing the mixture into a tablet. The punch then retracts, while a punch below the dye cavity rises into the cavity and ejects the tablet.
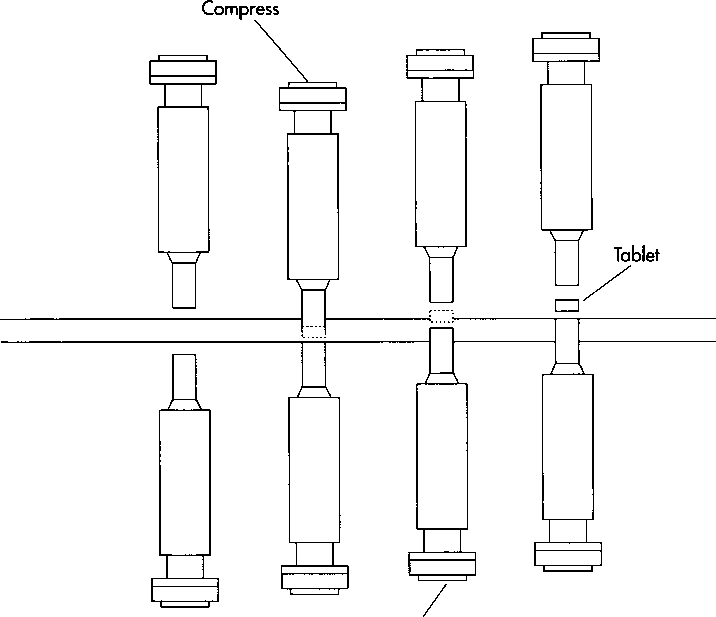
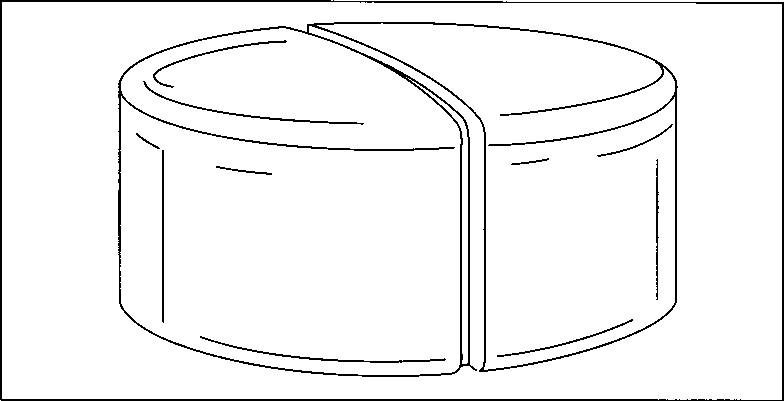
This drawing illustrates the principle of compression in a single-punch machine. First, the aspirin mixture is fed into a dye cavity. Then, a steel punch descends into the cavity and compresses the mixture into a tablet. As the punch retracts, another punch below the cavity rises to eject the tablet.
• As the feed shoe returns to fill the dye cavity again, it pushes the compressed tablet from the dye platform.
On rotary tablet machines, the mixture runs through a feed line into a number of dye cavities which are situated on a large steel plate. The plate revolves as the mixture is dispensed through the feed line, rapidly filling each dye cavity. Punches, both above and below the dye cavities, rotate in sequence with the rotation of the dye cavities. Rollers on top of the upper punches press the punches down onto the dye cavities, compressing the mixture into tablets, while roller-activated punches beneath the dye cavities lift up and eject the tablets from the dye platform.
Testing
6 The compressed tablets are subjected to a tablet hardness and friability test, as well as a tablet disintegration test (see Quality Control section below).
Bottling and packaging
7 The tablets are transferred to an automated bottling assembly line where they are dispensed into clear or color-coated poly-ethylene or polypropylene plastic bottles or glass bottles. The bottles are topped with cotton packing, sealed with a sheer aluminum top, and then sealed with a plastic and rubber child-proof lid. A sheer, round plastic band is then affixed to the circular edge of the lid. It serves as an additional seal to discourage and detect product tampering.
8 The bottles are then labeled with product information and an expiration date is affixed. Depending on the manufacturer, the bottles are then packaged in individual card- board boxes. The packages or bottles are then boxed in larger cardboard boxes in preparation for distribution to distributors
Finished aspirin tablets often have a line “scored” down the center so that the tablet can be broken into two parts with ease
Quality Control
Maintaining a high degree of quality control is extremely important in the pharmaceutical manufacturing industry, as well as required by the Food and Drug Administration (FDA). All machinery is sterilized before beginning the production process to ensure that the product is not contaminated or diluted in any way. In addition, operators assist in maintaining an accurate and even dosage amount throughout the production process by performing periodic checks, keeping meticulous batch records, and administering necessary tests. Tablet thickness and weight are also controlled.
Once the tablets have been produced, they undergo several quality tests, such as tablet hardness and friability tests. To ensure that the tablets won’t chip or break under normal conditions, they are tested for hardness in a machine such as the Schleuniger (or Heberlein) Tablet Hardness Tester. They are also tested for friability, which is the ability of the tablet to withstand the rigors of packaging and shipping. A machine called a Roche Friabilator is used to perform this test. During the test, tablets are tumbled and exposed to repeated shocks.
Another test is the tablet disintegration test. To ensure that the tablets will dissolve at the desirable rate, a sample from the batch is placed in a tablet disintegration tester such as the Vanderkamp Tester. This apparatus consists of six plastic tubes open at the top and bottom. The bottoms of the tubes are covered with a mesh screen. The tubes are filled with tablets and immersed in water at 37 degrees Fahrenheit (2.77 degrees Celsius) and retracted for a specified length of time and speed to determine if the tablets dissolve as designed.
Where To Learn More
Books
HIJSA’S Pharmaceutical Dispensing, 6th edition, Mack Publishing Company, 1966.
History of Pharmacy, 4th edition, The American Institute of History of Pharmacy, 1986.
An Introduction to Pharmaceutical Formu¬lation, Pergamon Press, 1965.
Mann, Charles C. The Aspirin Wars: Money, Medicine & One Hundred Years of Rampant Competition. Alfred A. Knopf, Inc. 1991.
Remington’s Pharmaceutical Sciences, 17th edition, Mack Publishing, 1985.
Periodicals
Draper, Roger. “A Pharmaceutical Cinder¬ella (History of Aspirin),” The New Leader. January 13, 1992, p. 16.
Weissmann, Gerald. “Aspirin,” Scientific American. January, 1991, pp. 84-90.
Wickens, Barbara. “Aspirin: What’s in a Name?,” Maclean’s. July 16, 1990, p. 40.