Background
The soda bottle so common today is made of polyethylene terephthalate (PET), a strong yet lightweight plastic. PET is used to make many products, such as polyester fabric, cable wraps, films, transformer insulation, generator parts, and packaging. It makes up 6.4 percent of all packaging and 14 percent of all plastic containers, including the popular soft drink bottle. Accounting for 43 percent of those sold, PET is the most widely used soft drink container. Aluminum, a close second, is 34 percent, while glass, which used to be 100 percent of the bottles, is only a small percentage of those sold today.
Plastics were first made in the 1800s from natural substances that were characterized by having chains of molecules. When these substances were combined with other chemicals in the laboratory, they formed products of a plastic nature. While hailed as a revolutionary invention, early plastics had their share of problems, such as flammability and brittleness. Polyesters, the group of plastics to which PET belongs, were first developed in 1833, but these were mostly used in liquid varnishes, a far cry from the solid, versatile form they took later.
Purely synthetic plastics that were a vast improvement on earlier plastics arrived in the early 1900s, yet they still had limited applications. Experimentation continued, with most of the hundreds of new plastics created over the next several decades failing commercially. PET was developed in 1941, but it wasn’t until the early 1970s that the plastic soda bottle became a reality. Nathaniel C. Wyeth, son of well-known painter N. C. Wyeth and an engineer for the Du Pont Corporation, finally developed a usable bottle after much experimentation.
Wyeth’s crucial discovery was a way to improve the blow-molding technique of making plastic bottles. Blow molding is ancient, having been used in glass-making technology for approximately two thousand years. Making plastic bottles by blow molding didn’t happen until suitable plastics were developed around 1940, but production of these bottles was limited because of inconsistent wall thickness, irregular bottle necks, and difficulty in trimming the finished product. Wyeth’s invention of stretch blow molding in 1973 solved these problems, yielding a strong, lightweight, flexible bottle.
The overwhelming success of PET soda bottles—in 1991, more than eight billion bottles were manufactured in the U.S.—has resulted in a disposal problem, but recycling of the bottles is growing, and manufacturers are finding new ways to use recycled PET.
Raw Materials
PET is a polymer, a substance consisting of a chain of repeating organic molecules with great molecular weight. Like most plastics, PET is ultimately derived from petroleum hydrocarbons. It is created by a reaction between terephthalic acid (CsHiOi) and ethylene glycol (C2H6O2).
Terephthalic acid is an acid formed by the oxidation of paraxylene (CsHto), an aromatic hydrocarbon, using just air or nitric acid. Para-xylene is derived from coal tar and petroleum using fractional distillation, a process that utilizes the different boiling points of compounds to cause them to “fall out” at different points of the process. Ethylene glycol is derived from ethylene (C2H4) indirectly through ethylene oxide (C2H4O), a substance also found in antifreeze. Ethylene is a gaseous hydrocarbon that is present in petroleum and natural gas, but is usually derived industrially by heating ethane or an ethane-propane mixture.
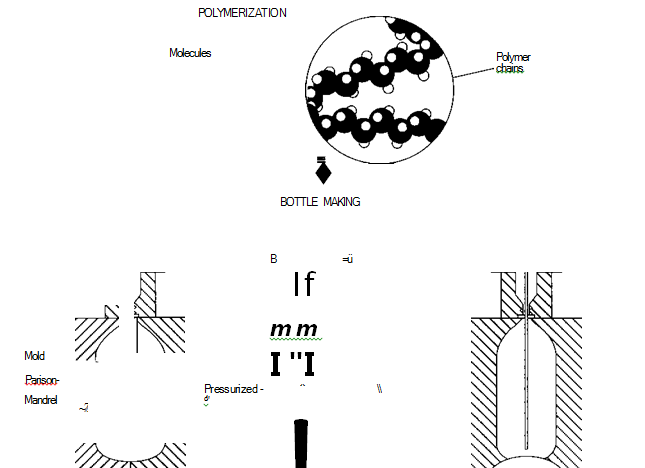
(In plastic soda bottle manufacture, ttie plastic—polyethylene terephthalate (PET)—is first polymerized, which involves creating long strings of molecules. Once the plastic is prepared, it undergoes stretch blow molding. In this process, a long tube (parison) of PET is put into a mold, and a steel rod (mandrel) is inserted into it. Next, highly pressurized air shoots through the mandrel and forces the parison against the walls of the mold. A separate bottom piece is inserted into the mold to shape the bottle so that it can stand on a flat surface.)
The Manufacturing Process
Polymerization
1 Before the bottles can be made, the PET itself must be manufactured, or polymerized. In polymerization, smaller molecules are combined to form larger substances. To make PET, terephthalic acid is first combined with methanol (CILOH). This reaction yields dimethyl terephthalate and water.
Next, the dimethyl terephthalate is combined with an excess of ethylene glycol at 305 degrees Fahrenheit (150 degrees Celsius) to yield another substance, bis 2-hydroxyethyl terephthalate and methanol.
2 The final step of polymerization involves the condensation polymerization of the bis 2-hydroxyethyl terephthalate. In this process, a polymer is formed while another molecule is released, or “falls out.” The condensation polymerization of bis 2-hydrox- yethyl terephthalate is carried out in a vacuum at 530 degrees Fahrenheit (275 degrees Celsius) and results in chains of PET and ethylene glycol (see step #1 above); the latter substance is continuously removed during polymerization and used to make more PET. After the PET mixture reaches the required viscosity (thickness), it is cooled to avoid In plastic soda bottle manufacture, ttie plastic—polyethylene terephtha- late (PET)—is first polymerized, which involves creating long strings of molecules. Once the plastic is prepared, it undergoes stretch blow molding. In this process, a long tube (parison) of PET is put into a mold, and a steel rod (mandrel) is inserted into it. Next, highly pressurized air shoots through the mandrel and forces the parison against the walls of the mold. A separate bottom piece is inserted into the mold to shape the bottle so that it can stand on a flat surface. degradation and discoloration. Later, it can be reheated for its various uses.
Bottle-making
3 PET beverage bottles are made using a process known as stretch blow molding (also called orientation blow molding). First, PET pellets are injection molded—heated and put into a mold—into a thin walled tube of plastic, called a parison. The parison is then cooled and cut to the proper length.
4 Next, the parison tube is re-heated and placed into another mold, which is shaped like a soda bottle, complete with screwtop. A steel rod (a mandrel) is slid into the parison. Highly pressurized air then shoots through the mandrel and fills the parison, pressing it against the inside walls of the mold. The pressure of the air stretches the plastic both radially (“out”) and axially (“down”). The combination of high temperature and stretching in the desired direction causes the molecules to polarize, line up and essentially crystallize to produce a bottle of superior strength. The entire procedure must be done quickly, and the plastic must be pressed firmly against the wall, or the bottle will come out misshapen. In order to give the bottom of the bottle its proper concave shape—so that it can stand upright—a separate bottom piece is attached to the mold during the blowing process.
5 The mold must then be cooled. Different cooling methods are used. Water in pipes may flow around the mold, or liquid carbon dioxide, highly pressurized moist air, or room air is shot into the bottle to cool it more directly. The procedure is preferably done quickly, to set the bottle before creep (flow) occurs.
6 The bottle is then removed from the mold. In mass production, small bottles are formed continuously in a string of attached botdes that are separated and trimmed. Other trimming must be done wherever the plastic leaked through the cracks of the mold (like the way pancake batter does when squeezed in a waffle maker). Ten to 25 percent of the plastic is lost this way, but it can be reused.
7 Some soft drink producers make their own bottles, but usually finished bottles are sent from specialty manufacturers to soft drink companies in trucks. Plastic is cheap to transport because it is light. Accessories such as lids and labels are manufactured separately. Occasionally, the plastic bottle manufacturer will put labels supplied by the soft drink company on the bottles before shipping them.
Quality Control
Polymerization is a delicate reaction that is difficult to regulate once the conditions are set and the process is set into motion. All molecules produced during the reaction, some of which might be side effects and impurities, remain in the finished product. Once the reaction gets going, it’s impossible to stop it at mid-point and remove impurities, and it is also difficult and expensive to eliminate unwanted products when the reaction is complete. Purifying polymers is an expensive process, and quality is hard to determine. Variations in the polymerization process could make changes that are undetectable in routine control tests.
The polymerization of terephthalic acid and ethylene glycol can yield two impurities: diethylene glycol and acetaldehyde. The amount of diethylene glycol is kept to a minimum, so that PET’s final properties are not affected. Acetaldehyde, which is formed during the polymerization as well as during the production of the bottle, will give a funny taste to the soft drink if it occurs in large enough amounts. By using optimum injection-molding techniques that expose the polymer to heat for a short time, very low concentrations of acetaldehyde appear and the taste of the beverage will be unaffected.
Testing is performed on those specific characteristics of PET that make it perfect for beverage bottles. Numerous standards and tests have been developed for plastics over the years. For instance, PET must be shatterproof under normal conditions, so bottles undergo impact resistance tests that involve dropping them from a specific height and hitting them with a specified force. Also, the bottle must hold its shape as well as resist pressure while stacked, so resistance to creep is measured by testing for deformity under pressure. In addition, soft drinks contain carbon dioxide; that’s what gives them their fizz. If carbon dioxide were able to escape through the bottle’s plastic walls, most beverages bought would have already gone flat. Hence, the bottle’s permeability to carbon dioxide is tested. Even its transparency and gloss are tested. All tests aim for consistency of size, shape, and other factors.
Recycling
A large number of the billions of PET bottles produced every year are thrown away, producing a serious environmental concern. Action has already been taken to stem the waste flow, mainly in the area of recycling. Only aluminum fetches a higher price at the recycling center than PET, so, at a one to two percent recovery rate, PET is the most extensively recycled plastic. Products made from recycled PET bottles include carpeting, concrete, insulation, and automobile parts. Still, it wasn’t until 1991 that the first PET soda bottle using recycled PET appeared. Consisting of 25 percent recycled PET, the bottle was introduced by Coca-Cola and Hoechst Celanese Corporation for use in North Carolina. By 1992, this bottle was being used in 14 other states, and other manufacturers (such as Pepsi, in partnership with Constar International Inc.) had produced a similar bottle.
Despite PET’s high recycling rate compared to other plastics, many companies and officials want to make it even higher. Current plans are to look into PET incineration, in which it is claimed that, if done properly, the products of complete combustion are merely carbon dioxide and water. Current goals of state and federal governments are that 25 to 50 percent of PET be recycled, that recycling of PET be made available to one-half of the United States population, and that 4000 curb- side recycling programs be implemented in the near future. In 1990, according to the National Association for Plastic Container Recovery, there were 577 curbside programs for PET.
Where To Learn More
Books
Beck, Ronald D. Plastic Product Design. Van Nostrand Reinhold, 1970.
Kaufman, Morris. Giant Molecules: The Technology of Plastics, Fibers, and Rubber. Doubleday, 1968.
Modern Plastics Encyclopedia, 1981-82. McGraw-Hill, 1981.
Richardson, Terry A. Industrial Plastics: Theory and Application. South-Western Pub¬lishing, 1983.
Wolf, Nancy and Ellen Feldman. Plastics: America’s Packaging Dilemma. Island Press, 1991.
Periodicals
“Picked Up, Dropped Off.” Beverage World. August, 1992, p. 16.
Kirkman, Angela and Charles H. Kline. “Recycling Plastics Today.” Chemtech. October, 1991, pp. 606-614.
Sfiligoj, Eric. “Answering the Critics: Recyclable Polyethylene Terephthalate Beverage Containers Are Replacing Glass Bottles.” Beverage World. June, 1992, p. 34.